Fonctionnalités
Le Logiciel Omni-Assistant, l’ultime SMQ (QMS) pour le secteur de la santé.
Un logiciel. Une solution. 15 modules entièrement intégrés.
Le logiciel Omni-Assistant est pleinement intégré, optimisé et hautement intuitif. Il vous aidera à appliquer et maintenir les meilleures pratiques, demeurer en conformité totale avec les normes et standards et à répondre aux organismes de réglementation.
Automatisation des flux de travail
Système d'automatisation des flux de travail de grade entreprise
L'automatisation des flux de travail est le module prédominant qui relie et connecte avec succès tous les modules. Permettant une intégration transparente, les processus sont activés d'un module à l'autre en fonction de règles métier spécifiques. La conception robuste du moteur de flux de travail lance efficacement les processus et formule des déclencheurs et des événements en fonction des besoins de votre entreprise.
.png)
Quelques exemples d’utilisation :
- Automatisez efficacement l'acheminement, la livraison et l'approbation de vos documents à des utilisateurs ou groupes spécifiques.
- Initiez un processus automatisé de gestion des non-conformités ou de ACAP, ou faites le déclencher par d'autres modules.
- Modèles électroniques de tâches et de liste d’items à compléter, éliminant le papier et garantissant l'efficacité.
- Gestion des priorités, planification des tâches, outils d'assignation et gestion des alertes.
- Réactions automatisées : alertes envoyées via des tâches, des e-mails, des messages Omni-Assistant et des SMS/messages texte.
Gestion documentaire
Automatisez les processus de contrôle des documents
La gestion manuelle de la documentation est laborieuse, coûteuse et difficile. Ces tâches sont encore plus compliquées étant donné la nécessité de régir les politiques et les procédures de conformité en matière d’accréditation dans le secteur de la santé. L’absence d’une gestion centralisée pour accéder et stocker les documents exacerbe ces problèmes.
Le module de gestion documentaire transforme les processus manuels en une solution entièrement numérique fournissant un cadre simple et efficace pour le partage, la révision, l'approbation, la publication réduisant le temps de délais d'exécution. Offrir un référentiel centralisé avec des modèles et une indexation des documents réduit considérablement le temps consacré au formatage des documents. Le flux de travail entièrement personnalisable lance automatiquement un processus défini pour gérer efficacement toute la documentation, y compris les alertes, les notifications, les rappels et les accusés de lecture et formation.
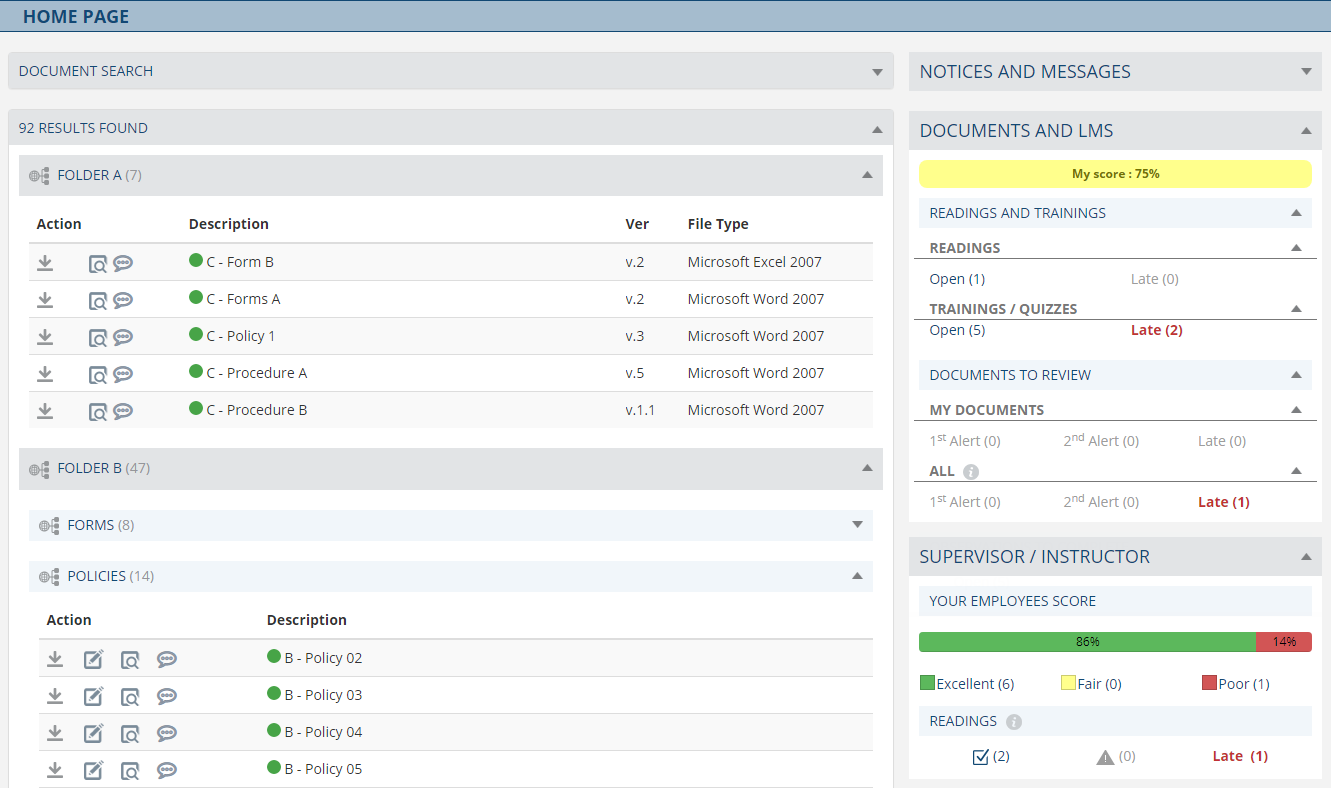
Fonctionnalités
- Suivi complet des versions, pistes d'audit et historique, y compris une capacité de recherche rapide et avancée.
- Flux de travail de révision et d’approbation efficaces et personnalisables.
- Processus d'approbation rapide adapté aux équipes de direction afin d'alléger leur charge de travail (par exemple votre directeur médical)
- Notifications, rappels et alertes en temps réel.
- Gestion efficace des exigences de lecture du personnel.
- Conversion et indexation PDF flexibles avec fonctionnalités de version contrôlée.
Gestion des actifs
Des équipements et des installations qualifiés en tout moment
La planification, la surveillance, le suivi des actifs (équipements et installations), la gestion des calendriers de maintenance, des contrats de service et des garanties, peuvent être fastidieux, difficiles et facilement négligés.
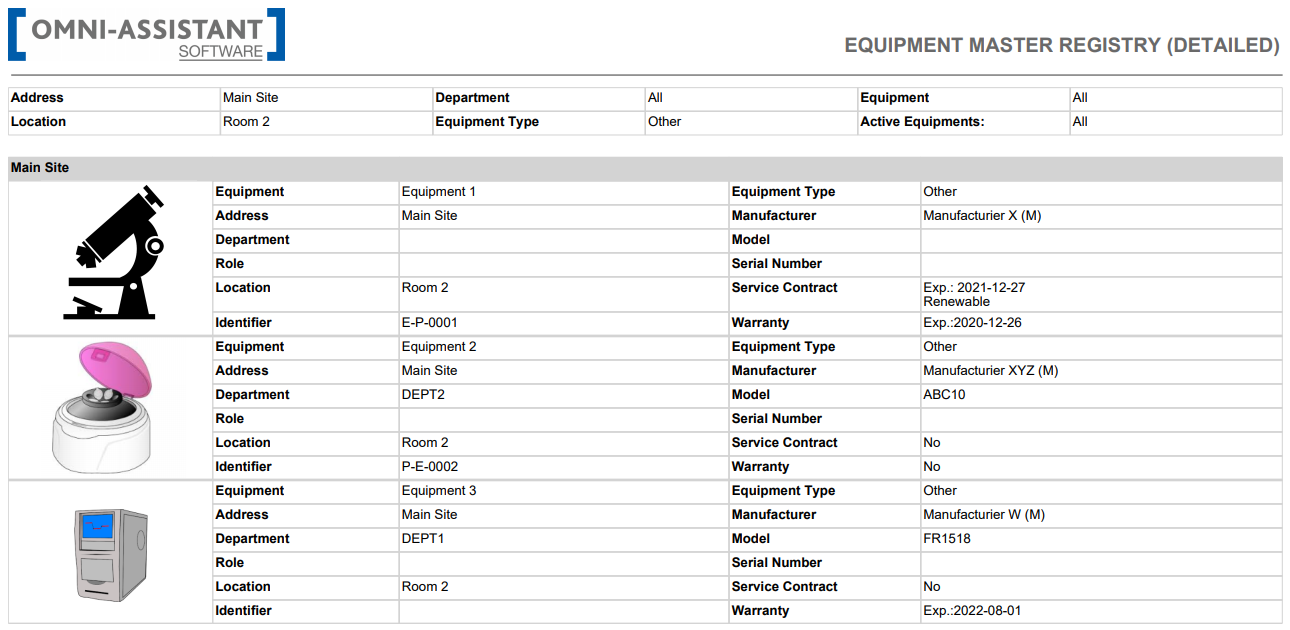
Le module gestion des actifs a été développé pour fournir sans effort et efficacement un processus standardisé et optimisé afin de faciliter la tenue de registres précis, la gestion des calendriers de maintenance, des contrats de service et des garanties de tout actif (équipements et installations) conformément aux réglementations.
features.module-3.p3
Fonctionnalités
- Registre des équipements (liste de vos équipements et de leur pédigrée).
- Contrôle total des changements, y compris le processus initial de qualification et de validation.
- Flux de travail d’étalonnage et de maintenance préventive entièrement personnalisables.
- Des fonctionnalités de sécurité avancées et spécialisées qui garantissent que le personnel sur le point d'effectuer un étalonnage ou une maintenance préventive est qualifié et connaît les SOP les plus récentes. Des enregistrements et alertes automatiques de non-conformité sont déclenchés s’ils se déroulent sans la formation la plus récente.
- Traçabilité complète et journaux d’audit.
- Gestion fiable des alertes. Création de rapports faciles pour fournir des informations précieuses sur les performances des actifs.
- Gabarits standardisés pour planifier des tâches de maintenance et d'étalonnage au besoin.
Gestion des points de contrôle
Le contrôle environnemental simplifié
La gestion des points de contrôle fait référence à la surveillance et à la capture des données de stockage et environnementales mesurées par divers appareils au sein de votre installation. Le processus manuel pour enregistrer la température dans vos réfrigérateurs, congélateurs, l'humidité ambiante ou la quantité de luminosité, etc. à partir de vos appareils prend du temps, est inefficace et sujet aux erreurs.
Le module Gestion des Points de Contrôle simplifie considérablement ce processus. Chaque mesure que vous devez enregistrer et documenter peut être configurée sans effort. Le moteur de flux de travail enregistre automatiquement toutes les mesures et points de contrôle tels que définis. L'intégration transparente avec d'autres modules lance des mises à jour complètes comprenant des e-mails, des alertes, des notifications de non-conformité, des inspections et des audits, y compris la gestion des risques. Le module s'intègre entièrement aux appareils de divers fabricants pour automatiser l'enregistrement des capteurs (sondes).
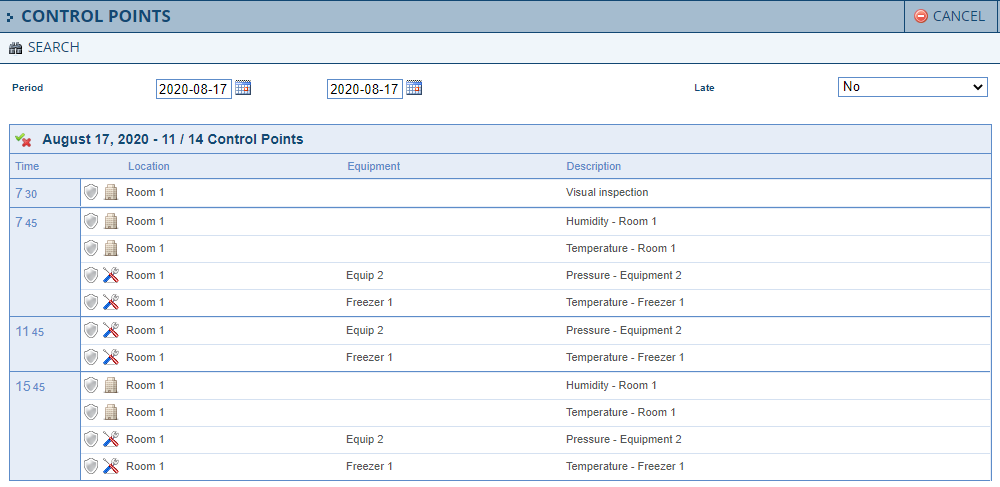
Fonctionnalités
- Omni-Assistant suit toutes les mesures dont vous avez besoin pour conserver une trace : nombre de bactéries, conductivité, humidité, luminosité, osmolalité, pression, résistivité, température et poids.
- Capacité à capturer les valeurs requises par des processus manuels ou automatisés.
- Intégration complète avec les dispositifs de détection de divers fabricants, notamment : Honeywell, Monit, Onset (hobo et inTemp), Cryopak (Mirador), Fourtec (Datanet) et Symco.
- Traçabilité complète liée au suivi du contrôle de température (chaîne du froid).
Gestion des non-conformités
Gestion des non-conformités efficace et standardisée
Lorsque des échecs se produisent, avec quelle facilité pouvez-vous identifier combien de cas ont été résolus et quelle a été l’action corrective ? Y a-t-il des tendances et cela s’est-il déjà produit ? Quels sont les processus et normes pour documenter les non-conformités ? Comment votre outil de gestion des événements non conformes se compare-t-il aux autres outils du marché ?
Le module de gestion des non-conformités vous fournit toutes les réponses et vous aide à obtenir des informations précieuses pour améliorer la qualité dans votre organisation. La solution de gestion des non-conformités d'Omni-Assistant est entièrement intégrée aux autres modules, permettant une gestion transparente des processus. Les mécanismes de surveillance et de conformité automatisés et efficaces maximisent les avantages tout en minimisant le coût des interventions manuelles et en éliminant les entrées de journalisation redondantes. Les modèles ACAP conviviaux et efficaces sont conçus pour capturer les échecs et les résolutions avec clarté. La transparence totale des processus ACAP permet au personnel responsable de garantir que le processus est géré efficacement et en temps opportun.
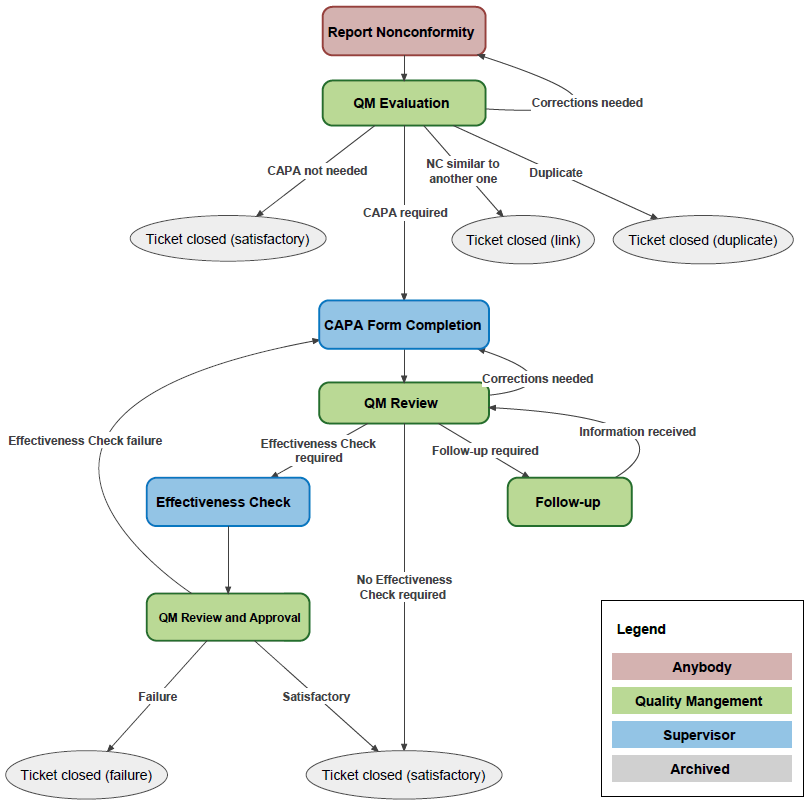
Fonctionnalités
- Une fois l'événement documenté, il l'achemine automatiquement en fonction de vos flux de travail jusqu'à ce qu'il soit entièrement résolu.
- Les ACAP non résolus sont automatiquement transmis à la direction.
- L’Omni-Assistant propose une liste de non-conformités standardisées que vous pouvez personnaliser.
- Les non-conformités et ACAP peuvent être entrés manuellement ou capturées automatiquement à partir d'autres modules.
- Flux de travail entièrement personnalisables.
Optimiseur de qualité pour les laboratoires
Réduisez les coûts liés à la mauvaise qualité
Le CdMQ « Coût de mauvaise qualité » se définit comme les coûts associés à un mauvais service ou de mauvais résultats. Les conséquences néfastes d'une mauvaise qualité ont un impact sur les opérations commerciales, augmentent les risques et peuvent entraîner de graves conséquences, à savoir des pertes financières, des duplications coûteuses, la fidélité des clients, une marque endommagée et des potentiellement des soins et traitements sous-optimaux . Réduire le coût d’une mauvaise qualité est l’un des meilleurs moyens d’augmenter l’efficacité de votre laboratoire et de réduire les coûts. La sélection de l'outil approprié est essentielle dans ce processus. Savez-vous quel est le coût d'une mauvaise qualité dans votre laboratoire ?
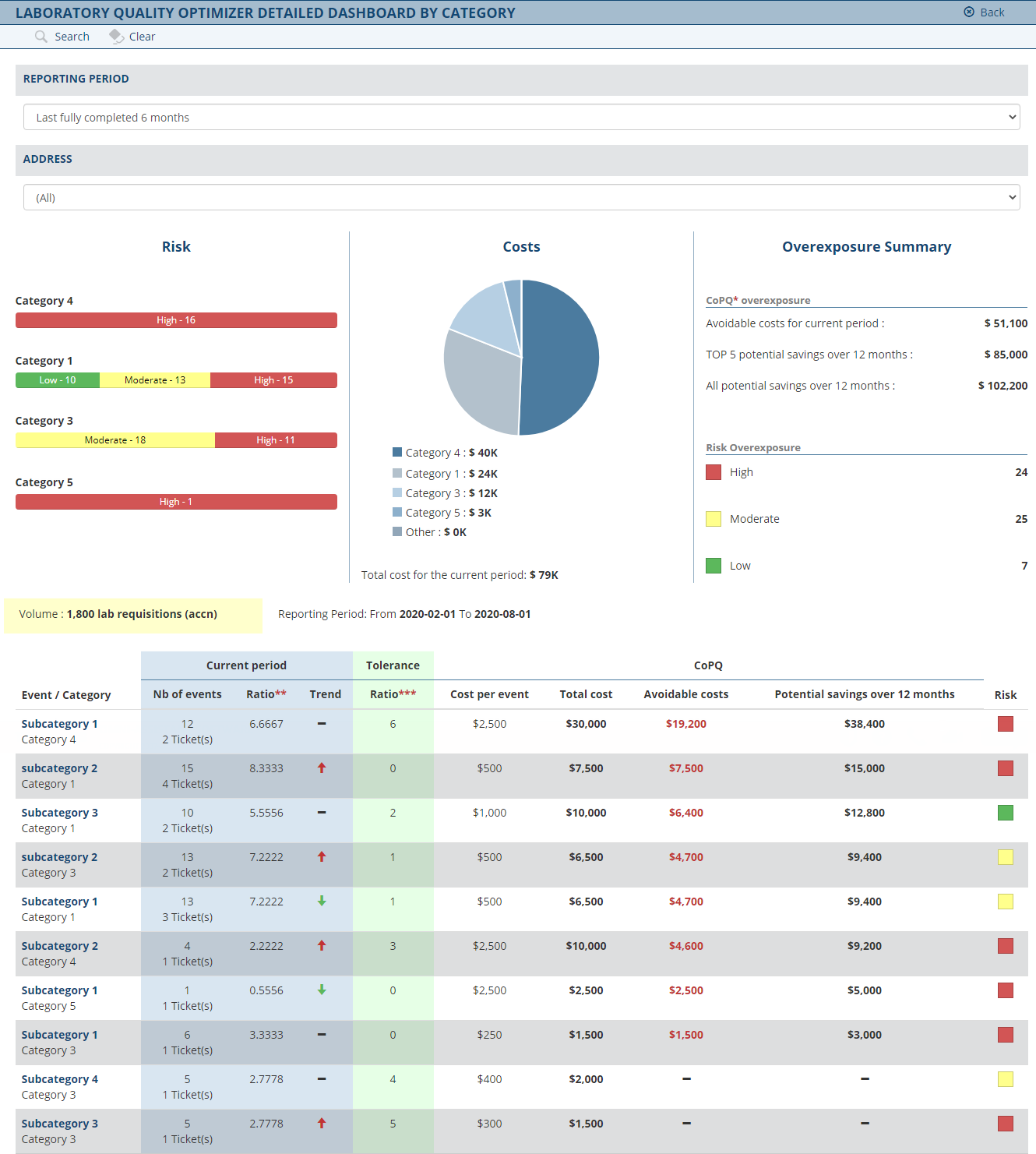
Le module Optimiseur de qualité pour les laboratoires analyse vos données et vous fournit une matrice des coûts quantifiés de mauvaise qualité (CdMQ). Les tableaux de bord interactifs montrent précisément où obtenir les économies potentielles les plus élevées.
Vous recevez des informations hiérarchisées qui vous permettent de prendre des mesures pour réaliser immédiatement des économies potentielles et vous recevez également une projection sur 12 mois de réductions des coûts opérationnels.
Fonctionnalités
- Matrice de calcul du coût de mauvaise qualité (CdMQ).
- Établissez des priorités en fonction des risques pour les patients et des économies potentielles.
- Compréhension claire et factuelle des événements de non-conformité et des coûts associés.
Gestion de la relation client
Vos clients sont importants, veillons à ce qu'ils soient satisfaits
Comment gérer efficacement les problèmes et les réclamations des clients ? Disposez-vous d'un outil central unifié pour identifier, hiérarchiser et résoudre rapidement les problèmes, ainsi que pour répondre de manière proactive aux préoccupations des clients ? En documentant les plaintes de vos clients et en les résolvant en temps opportun, un négatif peut devenir positif et entraîner une amélioration du service client, de la fidélité et de la qualité de la livraison.
L’objectif du module de gestion de la relation client est simple: résoudre efficacement tous les problèmes grâce à des processus automatisés pour améliorer la qualité et garantir la conformité réglementaire.
Fonctionnalités
- Gestion des alertes pour garantir un traitement efficace et rapide de toute préoccupation.
- Gestion des priorités, de la planification des tâches et de l’assignation.
- Traçabilité complète avec journal historique.
- Rapports et statistiques pour évaluer les progrès.
Gestion de la qualification des fournisseurs
Qualifier vos fournisseurs de manière intelligente
Même si une évaluation efficace des fournisseurs confirme leur capacité, leur durabilité et garantit leur engagement envers la qualité, elle peut être décousue et difficile à gérer.
Le module de gestion de la qualification des fournisseurs réduit le temps passé à valider et approuver manuellement les fournisseurs préférés. Le statut des fournisseurs, y compris la possibilité de stocker des enregistrements et des notes de documentation supplémentaires, est facilement disponible pour votre examen.
Il fournit un programme de surveillance pour confirmer que ces fournisseurs fonctionnent efficacement et fournissent des produits et services de haute qualité. Des informations précieuses sont obtenues sur les qualifications de votre fournisseur en examinant le tableau de bord de statut.
Fonctionnalités
- Flux de qualification entièrement personnalisables.
- Examens périodiques planifiés avec alertes d’examen et alertes d’escalade.
- Tableau de bord du statut de qualification des fournisseurs.
- Rapports de preuves et statistiques facilement disponibles.
Gestion du risque
Une gestion du risque qui apporte des résultats
Le risque fait partie intégrante de toute entreprise et il incombe à la direction de mettre en place un plan de gestion des risques. Si le risque n’est pas géré efficacement, il peut affecter les soins aux patients, la sécurité du personnel, l’environnement de travail et la durabilité de l’organisation. La sélection d'outils, de méthodologies, de contrôles et de pratiques pour identifier, analyser et gérer efficacement les risques est essentielle, mais peut être compliquée et difficile à suivre.
Le module Gestion du risque vous propose une large gamme d'outils pratiques et faciles à suivre pour identifier, mesurer et mettre en œuvre efficacement des contrôles d'atténuation des risques. Doté d'une analyse d'évaluation des risques facile à comprendre, ce module soutient vos actions pour réduire les risques, mettre en œuvre des améliorations et prendre des décisions éclairées. La fourniture d'une identification bien définie des situations dangereuses, des dommages possibles, du degré de probabilité et de gravité, soutient la création d'une matrice des risques pour gérer les risques au sein de votre organisation.
Fonctionnalités
- Outil dynamique qui bâti votre matrice de risque, vous offrant une visibilité sur les risques connus.
- Documentation des mécanismes de contrôle et des améliorations potentielles.
- Examens périodiques programmés, y compris des alertes et des alertes d'escalade.
- Vaste bibliothèque de risques de laboratoire.
Gestion des compétences du personnel
Gestion digitale des compétences et de l’apprentissage
De nombreuses organisations améliorent leurs opérations en acquérant un système de gestion de l'apprentissage (LMS / ENA) qui devient partie intégrante de leur activité. La formation, la qualification, la certification et les compétences des employés constituent un avantage commercial important tout en étant rentables en termes de temps et d'argent. La sélection du meilleur système de gestion de l'apprentissage pour votre entreprise est essentielle, en particulier dans le secteur de la santé, où une conformité réglementaire est requise.
Le module de Gestion des compétences du personnel offre bien plus que des produits LMS traditionnels. Le processus garantit l'attribution et le suivi de la formation du personnel, y compris l'attribution de cours séquentiels afin d'atteindre la compétence du personnel et le respect de l'accréditation. L'intégration transparente à d'autres modules vous permet de facilement lier vos politiques et procédures. Vous contrôlez totalement la sélection des cours, des évaluations et du matériel de formation que vous choisissez d'ajouter à la solution eLearning. Les progrès de la formation des employés, les rappels et le suivi sont facilement disponibles.
L'évaluation des compétences au sein du module Gestion des compétences du personnel suit un cadre CLIA appelé « Big 6 », ce qui signifie que six (6) piliers doivent être complétés afin d'obtenir la certification. Il s’agit d’un différenciateur important pour garantir une évaluation complète des compétences et la formation de votre personnel.
Fonctionnalités
- Flux entièrement personnalisable.
- Visibilité complète sur l’état des compétences d’apprentissage des employés.
- Automatisez le BIG 6. Obtenez une plus grande uniformité et une qualité extraordinaire :
- Observations directes des tests de routine des patients.
- Suivi de l'enregistrement et du rendu des résultats des tests.
- Examen des enregistrements : examen des résultats de tests intermédiaires ou des feuilles de travail, des enregistrements QC, des résultats TC et des enregistrements de maintenance préventive.
- Maintenance : observations directes des performances de maintenance des instruments et des contrôles de fonctionnement.
- Évaluation des performances des tests en testant des échantillons préalablement analysés, des échantillons internes aveugles ou des échantillons TC externes.
- Évaluation des compétences en résolution de problèmes.
Archivage des échantillons
Laissez votre Omni-Assistant faire le travail
Les laboratoires traitent chaque année un grand nombre de différents types d’échantillons. Il est essentiel de savoir où se trouve chaque échantillon à un moment donné dans le laboratoire une fois la phase de test terminée.
Le stockage, la récupération et l'élimination des échantillons sont des étapes essentielles pour assurer la conformité, mais également très importantes pour l'efficacité du laboratoire, l'excellence opérationnelle et les soins aux patients.
Un processus manuel ou mal planifié retardera inévitablement la réalisation de cette tâche, aura un impact sur l’intégrité des échantillons et, en fin de compte, sur les soins aux patients.
Avec la solution d’archivage des échantillons, vous avez un contrôle total avec des informations précises sur l’emplacement de chaque échantillon. Le module d'archivage des échantillons de laboratoire réduit le temps de cycle du processus en cataloguant les échantillons en fonction de règles définies par l'utilisateur en fonction de vos processus. Par exemple; le stockage des échantillons en fonction de leurs exigences réglementaires en matière de conservation, la conservation des échantillons pour des tests répétés ou supplémentaires ainsi que la capacité de localiser facilement et efficacement les échantillons pour une élimination en toute sécurité. Une flexibilité et des informations complètes sont offertes à portée de main pour suivre sans effort les échantillons de laboratoire.
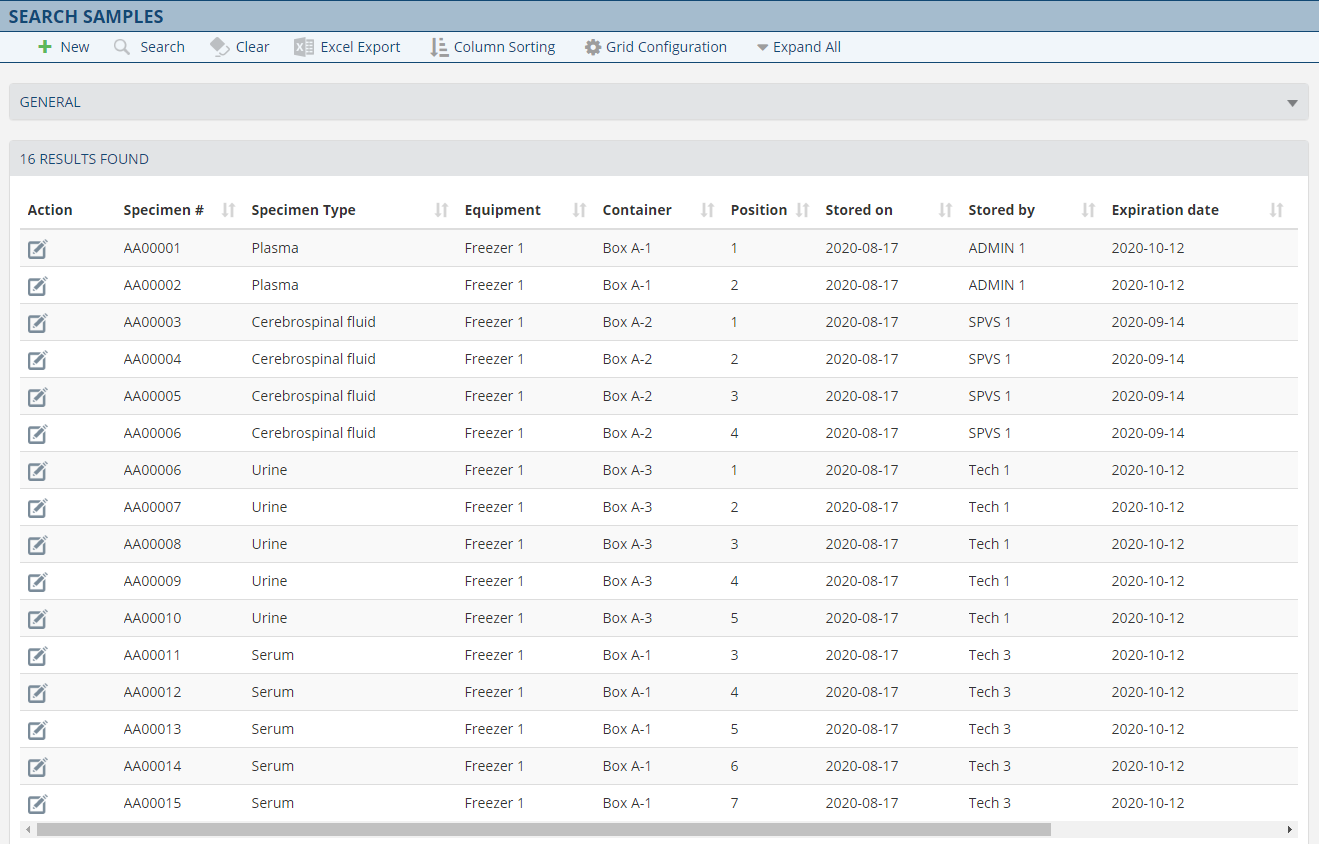
Fonctionnalités
- Archivez rapidement et efficacement vos échantillons.
- Identifier l’échantillon, le contenant d'archivage, le département et l'unité de stockage.
- Surveillance des exigences de conservation des échantillons en fonction des paramètres définis par l'utilisateur.
- Gestion efficace des purges.
- Recherche rapide et récupération accélérée des enregistrements.
Gestion de l’inventaire
Vos règles pour avoir les bons produits, en bonne quantité et aux bons endroits.
Le processus que vous suivez lors du stockage, de l’enregistrement, du suivi et de la gestion de votre inventaire est essentiel pour garantir un approvisionnement adéquat pour votre installation. Comment gérez-vous votre inventaire? Combien de fois avez-vous manqué de stock ou avez-vous fait rappeler un lot de réactifs ?
Le module de gestion de l’inventaire est conçu pour répondre spécifiquement aux besoins cliniques et de soins du secteur de la santé, inventaire optimisé et en toute conformité . Grâce à la lecture de codes-barres, suivez de manière transparente vos produits sur plusieurs sites et entrepôts. Avec une visibilité en temps réel des quantités, des lots et leurs expiration et la possibilité de définir des alertes, vous demeurez conforme et vos risques d’inventaires sont adressés.
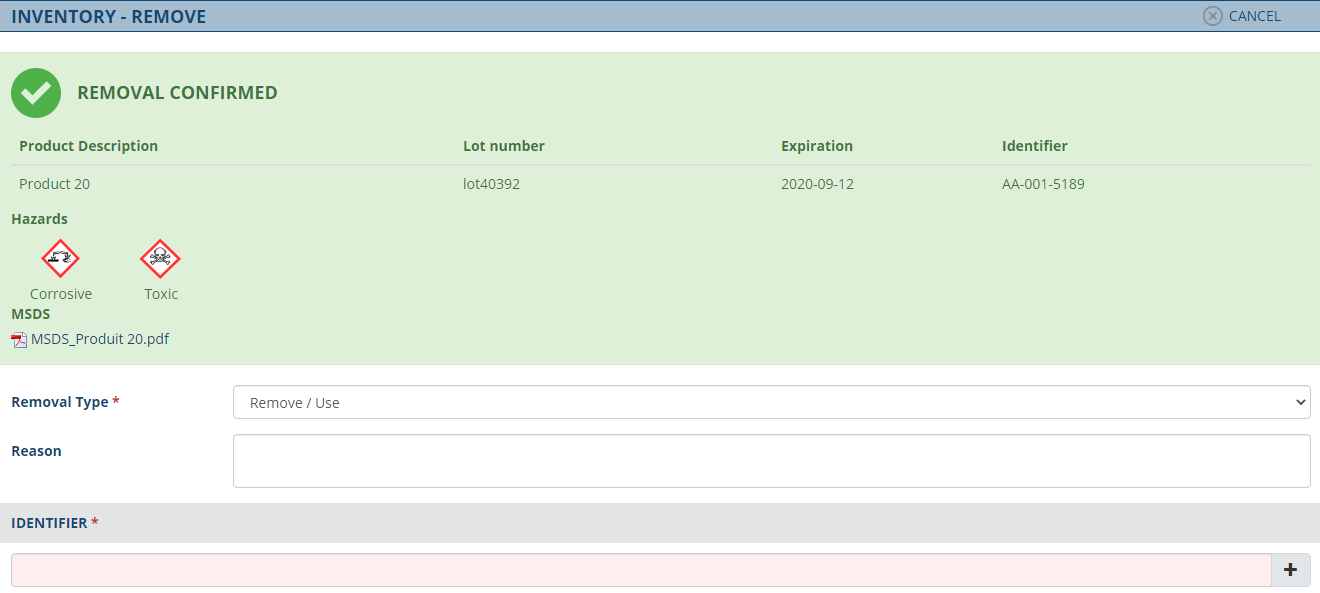
Fonctionnalités
- Traçabilité totale. Réception, transfert, utilisation et élimination des produits.
- Suivi des lots et des dates de péremption. Qualification, rappels et rotation des stocks.
- S'intégrer pleinement à Toxyscan pour la gestion des MSDS (Fiches de Données de Sécurité) / FDS (Fiches de Données de Sécurité).
- Alertes de réapprovisionnement. Alertes personnalisées pour les niveaux de stock faibles et critiques.
- Lorsqu'il est utilisé en combinaison avec le module de gestion des actifs et le module de gestion des points de contrôle, il permet de suivre les températures des réactifs (chaîne du froid) et d'alerter lorsqu'une température anormale est détectée ou affecte l'un de vos produits.
Gestion des inspections et des audits
Soyez prêt pour une inspection à tout moment.
Rester en conformité avec les diverses réglementations et normes de qualité est un défi commercial permanent. Êtes-vous prêt à être audité? De combien de listes de contrôle de conformité et de versions disposez-vous ? Avez-vous résolu toutes les non-conformités découvertes précédemment avec des preuves appuyées ? et documentation ? Ce ne sont là que quelques-unes des questions auxquelles le personnel du secteur de la santé et des laboratoires doit répondre lors de la planification d’inspections ou d’audits.
Le module de gestion des inspections et des audits introduit une nouvelle approche qui garantit que vous êtes prêt pour l'inspection à tout moment. Grâce à un processus innovant et intégré, il se connecte de manière transparente aux autres modules Omni-Assistant et génère sans effort et automatiquement des preuves de conformité pour vous, optimisant ainsi considérablement votre temps.
Vous contrôlez totalement les inspections et les audits internes ou externes, et êtes en mesure de réduire considérablement le temps, les coûts et les ressources nécessaires pour mettre en valeur votre conformité et votre excellence opérationnelle.
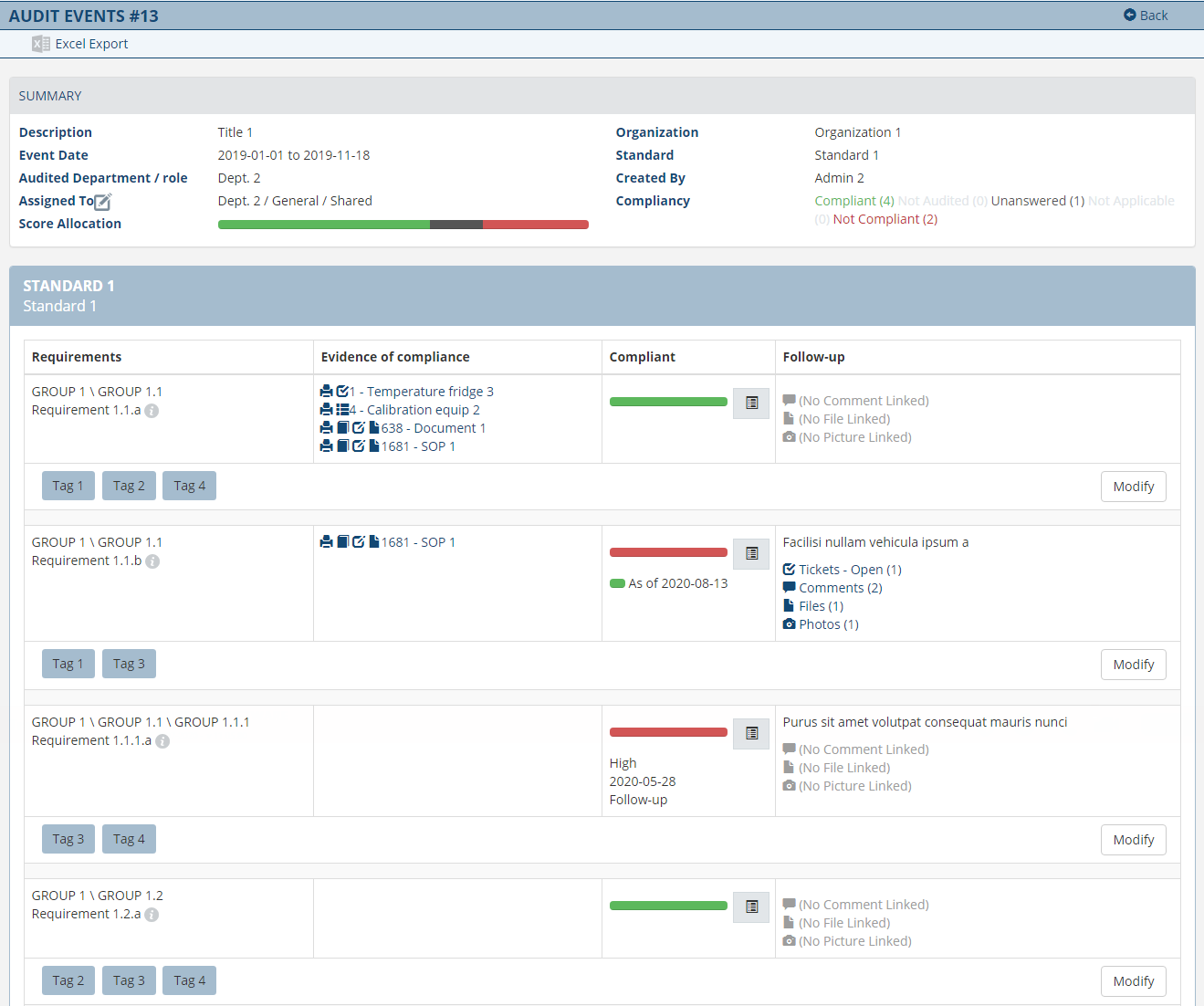
Fonctionnalités
- Disposez d’une image claire et complète de votre situation par rapport aux exigences d’inspection.
- Preuves de conformité générées automatiquement.
- Historisation de vos inspections et audits.
- Plus de 120 listes de contrôle maintenues à jour pour vous; CAP, CMS, CSTM, FDA, ISO 15189, ISO 17025, ISO 22870, WMDA et plus encore.
Répertoire des analyses de laboratoires
Répertoire des analyses avec une apparence et une convivialité qui mettent en valeur votre service
Votre répertoire des analyses doit toujours être à jour et permettre un accès facile aux informations sur les tests. Commander des tests de laboratoire appropriés fait partie intégrante du diagnostic, du suivi et du traitement des problèmes de santé. Fournir au personnel de votre laboratoire un processus efficace pour publier régulièrement des informations à jour sur les tests contribue à de bons soins aux patients. À quelle fréquence publiez-vous votre recueil de tests de laboratoire ? Avec quelle facilité ces informations peuvent-elles être consultées ?
Le module Répertoire des analyses de laboratoires vous permet de mettre en œuvre efficacement une gestion du changement et de maintenir les informations à jour. Il optimise votre temps, partage les informations instantanément et améliore significativement la qualité de service.
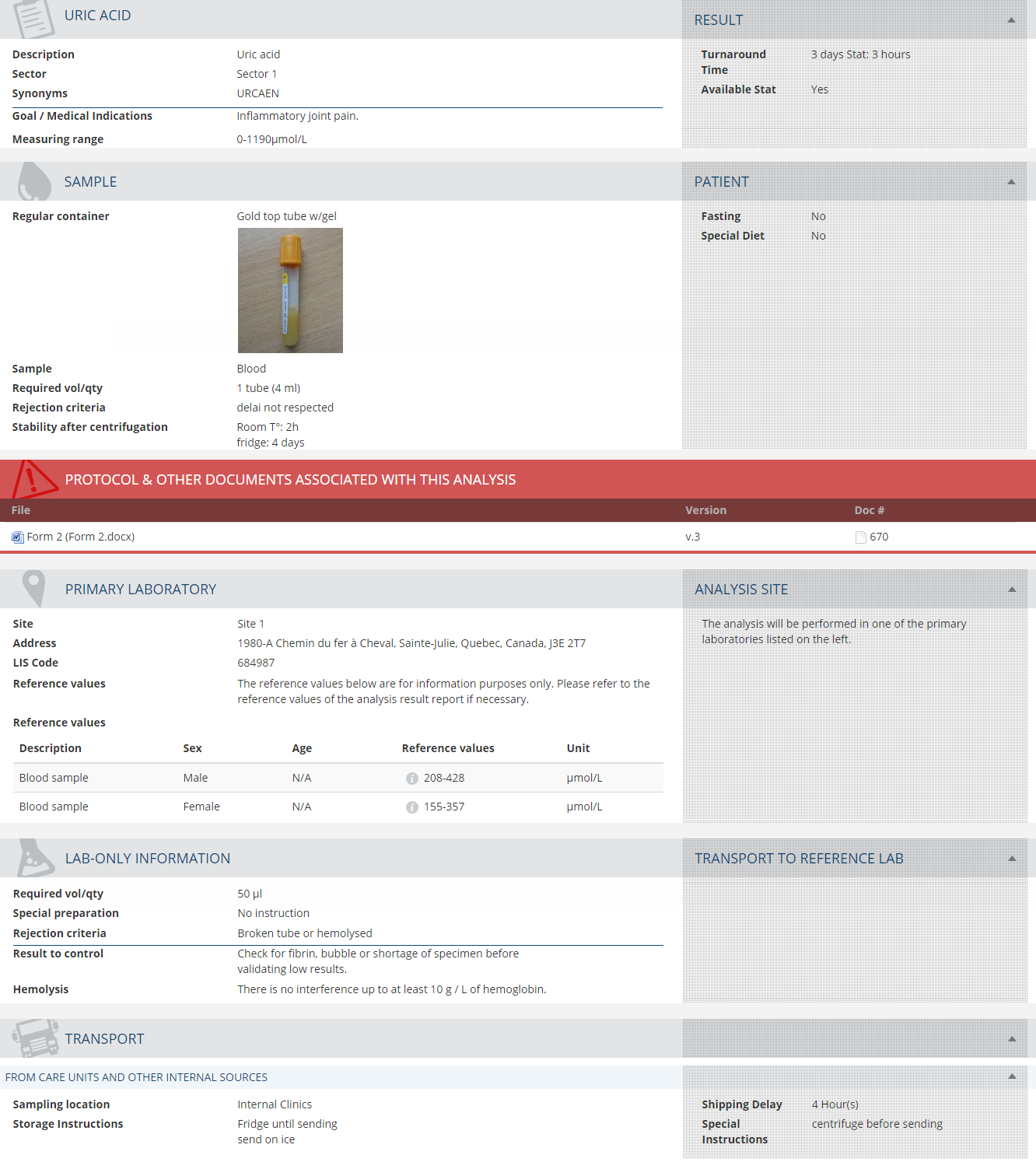
Fonctionnalités
- Portail public. Accédez et obtenez un dossier complet pour chaque analyse : abréviation, description, synonymes connus, raisons médicales pour commander l'analyse, échantillon et quantité requise, protocoles, règles de transport, délais prévus, entre autres.
- Portail interne (usage en laboratoire uniquement). Extrayez toutes les informations du portail public ainsi que toutes les règles et informations privées ou réservées au personnel du laboratoire.
- Contrôle total des changements.
